Introduction to Molecular Discovery
The process of discovering molecules that have the properties needed to create new medicines and materials is cumbersome and expensive, consuming vast computational resources and months of human labor to narrow down the enormous space of potential candidates. Large language models (LLMs) like ChatGPT could streamline this process, but enabling an LLM to understand and reason about the atoms and bonds that form a molecule, the same way it does with words that form sentences, has presented a scientific stumbling block.
A Promising Approach
Researchers from MIT and the MIT-IBM Watson AI Lab created a promising approach that augments an LLM with other machine-learning models known as graph-based models, which are specifically designed for generating and predicting molecular structures. Their method employs a base LLM to interpret natural language queries specifying desired molecular properties. It automatically switches between the base LLM and graph-based AI modules to design the molecule, explain the rationale, and generate a step-by-step plan to synthesize it.
How it Works
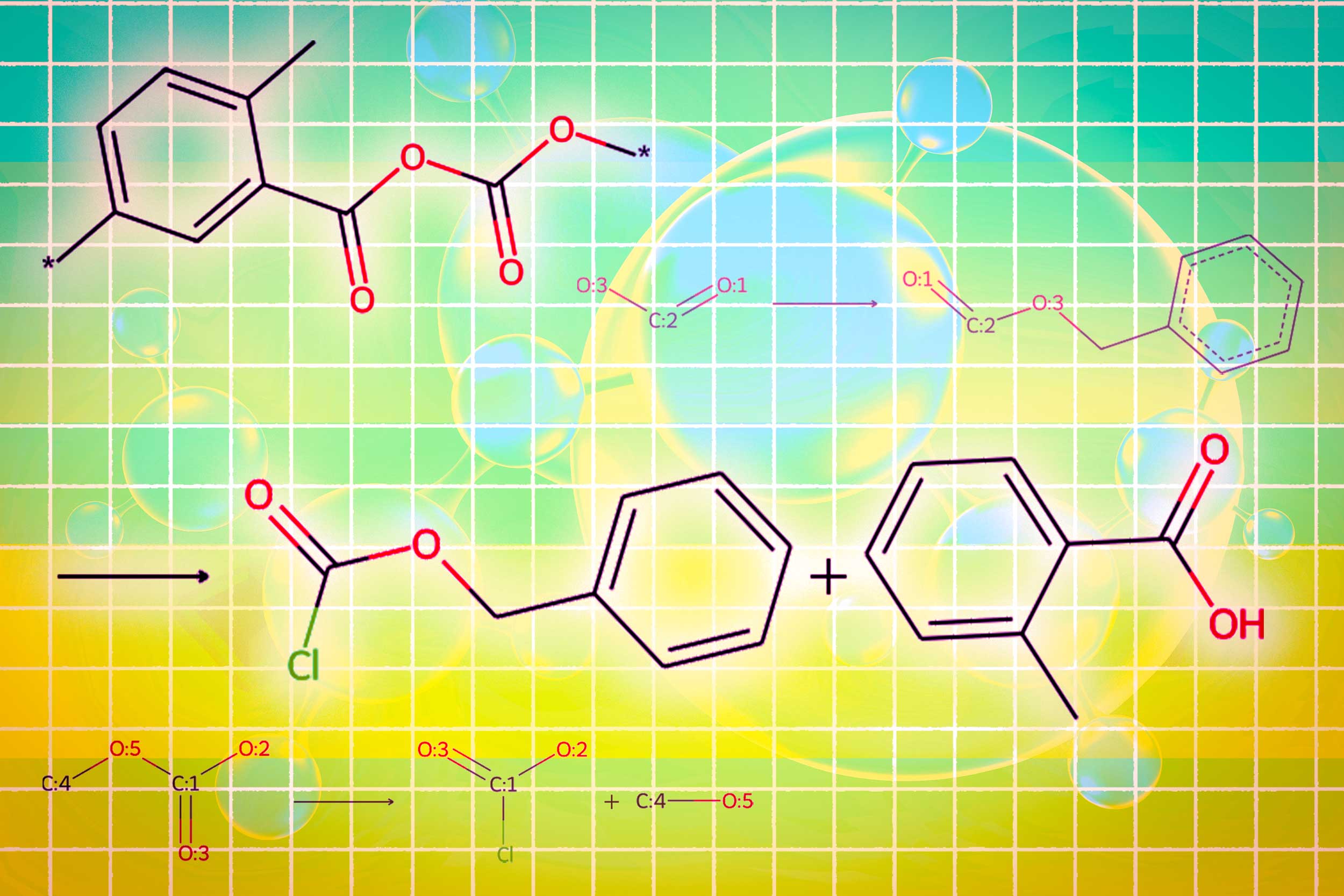
The method, called Llamole, uses a base LLM as a gatekeeper to understand a user’s query — a plain-language request for a molecule with certain properties. For instance, perhaps a user seeks a molecule that can penetrate the blood-brain barrier and inhibit HIV, given that it has a molecular weight of 209 and certain bond characteristics. As the LLM predicts text in response to the query, it switches between graph modules. One module uses a graph diffusion model to generate the molecular structure conditioned on input requirements. A second module uses a graph neural network to encode the generated molecular structure back into tokens for the LLMs to consume. The final graph module is a graph reaction predictor which takes as input an intermediate molecular structure and predicts a reaction step, searching for the exact set of steps to make the molecule from basic building blocks.
Benefits of Llamole
When compared to existing LLM-based approaches, this multimodal technique generated molecules that better matched user specifications and were more likely to have a valid synthesis plan, improving the success ratio from 5 percent to 35 percent. It also outperformed LLMs that are more than 10 times its size and that design molecules and synthesis routes only with text-based representations, suggesting multimodality is key to the new system’s success.
Better, Simpler Molecular Structures
In the end, Llamole outputs an image of the molecular structure, a textual description of the molecule, and a step-by-step synthesis plan that provides the details of how to make it, down to individual chemical reactions. In experiments involving designing molecules that matched user specifications, Llamole outperformed 10 standard LLMs, four fine-tuned LLMs, and a state-of-the-art domain-specific method. At the same time, it boosted the retrosynthetic planning success rate from 5 percent to 35 percent by generating molecules that are higher-quality, which means they had simpler structures and lower-cost building blocks.
Conclusion
The researchers hope that Llamole will be an end-to-end solution where, from start to finish, they would automate the entire process of designing and making a molecule. If an LLM could just give you the answer in a few seconds, it would be a huge time-saver for pharmaceutical companies. In future work, the researchers want to generalize Llamole so it can incorporate any molecular property. In addition, they plan to improve the graph modules to boost Llamole’s retrosynthesis success rate.
FAQs
- What is Llamole?: Llamole is a method that uses a large language model (LLM) and graph-based AI models to design molecules and generate step-by-step synthesis plans.
- How does Llamole work?: Llamole uses a base LLM to interpret natural language queries and switches between graph modules to design the molecule, explain the rationale, and generate a synthesis plan.
- What are the benefits of Llamole?: Llamole generated molecules that better matched user specifications and were more likely to have a valid synthesis plan, improving the success ratio from 5 percent to 35 percent.
- What are the future plans for Llamole?: The researchers want to generalize Llamole so it can incorporate any molecular property and improve the graph modules to boost Llamole’s retrosynthesis success rate.